NEWS
最新消息
FDA Breakthrough Therapy Designation for Pfizer's RSV Vaccine
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2022-03-04 15:52
- 访问量:
【概要描述】“Today’s decision is a pivotal next step in our path towards potential regulatory approval for our maternal RSV vaccine candidate and is an important milestone in our efforts to help address the detrimental impact RSV disease has on infants,” said Kathrin U. Jansen, Ph.D., Senior Vice President and Head of Vaccine Research & Development at Pfizer Inc. “If approved by the FDA, this maternal immunization has the potential to be the first vaccine candidate to help protect infants in their vulnerable first months of life from disease caused by this highly-contagious virus. We look forward to our ongoing dialogue with the FDA to accelerate the development of our maternal RSV vaccine candidate.”
FDA Breakthrough Therapy Designation for Pfizer's RSV Vaccine
【概要描述】“Today’s decision is a pivotal next step in our path towards potential regulatory approval for our maternal RSV vaccine candidate and is an important milestone in our efforts to help address the detrimental impact RSV disease has on infants,” said Kathrin U. Jansen, Ph.D., Senior Vice President and Head of Vaccine Research & Development at Pfizer Inc. “If approved by the FDA, this maternal immunization has the potential to be the first vaccine candidate to help protect infants in their vulnerable first months of life from disease caused by this highly-contagious virus. We look forward to our ongoing dialogue with the FDA to accelerate the development of our maternal RSV vaccine candidate.”
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2022-03-04 15:52
- 访问量:
FDA Breakthrough Therapy Designation for Pfizer's RSV Vaccine
Respiratory syncytial virus (RSV) belongs to the genus Pneumovirus of the family Paramyxoviridae and has only one serotype. It mainly causes lower respiratory tract infections such as bronchiolitis and pneumonia in infants under 6 months old, and upper respiratory tract infections such as rhinitis and colds in older children and adults. The virus is highly contagious and occurs in winter and early spring. It is mainly transmitted by droplets, or by contaminated hands and surfaces. It first proliferates in nasopharyngeal epithelial cells, and then spreads to the lower respiratory tract. The incubation period The virus is released in about 4-5 days and can last for 1-5 weeks.
For most young people, symptoms can resemble the common cold, but for infants, the immunocompromised, and the elderly, it can be life-threatening. However, so far, there are no effective treatments and approved preventive vaccines specifically for RSV. RSV can be divided into two subtypes based on antigenicity: Type A and Type B. Pfizer’s RSV vaccine candidate contains two RSV prefusion F glycoproteins, designed to provide the best possible response to RSV A and RSV B. Protect. Research by the National Institutes of Health (NIH) has shown that antibodies specific for the prefusion F protein are highly effective at blocking viral infection.

On March 2, 2022,Pfizer announced that its respiratory syncytial virus (RSV) vaccine candidate, PF-06928316 or RSVpreF, received Breakthrough Therapy Designation from the US Food and Drug Administration (FDA) for prevention of RSV-associated lower respiratory tract illness in infants from birth up to six months of age by active immunization of pregnant women. The FDA’s Breakthrough Therapy Designation is designed to expedite the development and review of drugs and vaccines that are intended to treat or prevent serious conditions and preliminary clinical evidence indicates that the drug or vaccine may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s).
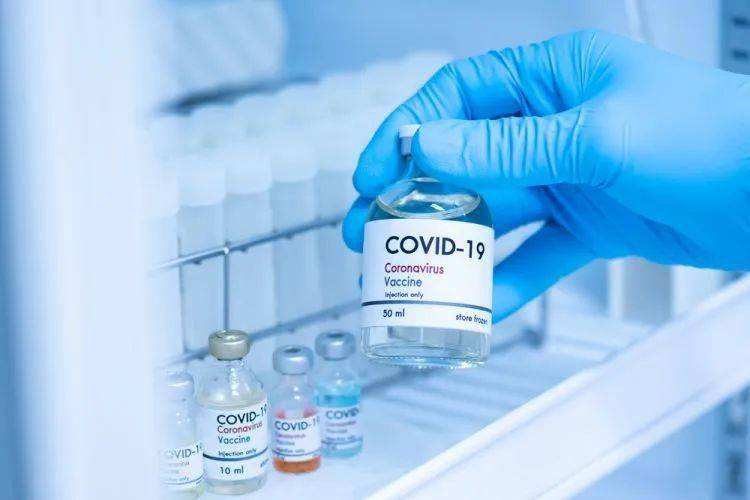
“Today’s decision is a pivotal next step in our path towards potential regulatory approval for our maternal RSV vaccine candidate and is an important milestone in our efforts to help address the detrimental impact RSV disease has on infants,” said Kathrin U. Jansen, Ph.D., Senior Vice President and Head of Vaccine Research & Development at Pfizer Inc. “If approved by the FDA, this maternal immunization has the potential to be the first vaccine candidate to help protect infants in their vulnerable first months of life from disease caused by this highly-contagious virus. We look forward to our ongoing dialogue with the FDA to accelerate the development of our maternal RSV vaccine candidate.”


美国联邦巡回上诉法院表示娇生公司 全球畅销精神分裂症药物的专利可能无效

注射用醋酸地加瑞克专利无效口审将于本月底进行

外观专利如何进行海外布局?