NEWS
最新消息
Zealand Pharma's New Fast-acting Hypoglycemia Drug Approved by FDA
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2021-03-25 14:09
- 访问量:
【概要描述】On March 22, the Danish pharmaceutical company Zealand Pharma, established in 1998, announced that the FDA approved Dasiglucagon subcutaneous injections for the treatment of severe hypoglycemia in children over 6 years old and adults with diabetes. Hypoglycemia is caused by a severe drop in blood sugar levels, and severe hypoglycemia is generally an acute and life-threatening condition in which the blood sugar level suddenly drops when diabetic patients receive insulin therapy. Severe hypoglycemia is also the most typical complication in the clinical treatment of diabetes. It mainly occurs in type 1 diabetic patients and type 2 diabetic patients taking insulin. In the United States alone, approximately 300,000 people are hospitalized for the disease each year. Dasiglucagon is a world-first soluble stable analog of glucagon discovered and developed by Zealand. Glucagon is a hormone secreted by the islet α-cells of the pancreas. It is opposed to insulin and plays a role in increasing blood sugar. Zealand uses its unique peptide drug research platform to change some of the amino acids in wild-type glucagon to maintain its biological activity and make it have higher stability and solubility. Compared with the previously marketed lyophilized powder preparation therapeutic drugs, it can be pre-installed in the emergency pen and can be injected subcutaneously during clinical use, which is extremely convenient. The FDA approval was mainly based on the efficacy data of three randomized, double-blind, placebo-controlled, multi-center phase III trials conducted in adults with type 1 diabetes between the ages of 6 and 17 years. The data shows that whether it is an adult patient or a child patient, the median time for blood glucose levels to recover after receiving Dasiglucagon injection is 10 minutes, while that of the placebo control group is 30 to 45 minutes. In a phase III study for adults, 99% of hypoglycemia patients recovered from insulin-induced hypoglycemia within 15 minutes after using Dasiglucagon. The study confirmed the potential of Dasiglucagon to quickly and effectively rescue severe hypoglycemia in diabetic patients. Dasiglucagon expects to start marketing the drug in the United States in June, bringing new treatment options to diabetic patients. It must be said that this is an important milestone in the history of diabetic hypoglycemia treatment. Let us look forward to it!
Zealand Pharma's New Fast-acting Hypoglycemia Drug Approved by FDA
【概要描述】On March 22, the Danish pharmaceutical company Zealand Pharma, established in 1998, announced that the FDA approved Dasiglucagon subcutaneous injections for the treatment of severe hypoglycemia in children over 6 years old and adults with diabetes.
Hypoglycemia is caused by a severe drop in blood sugar levels, and severe hypoglycemia is generally an acute and life-threatening condition in which the blood sugar level suddenly drops when diabetic patients receive insulin therapy. Severe hypoglycemia is also the most typical complication in the clinical treatment of diabetes. It mainly occurs in type 1 diabetic patients and type 2 diabetic patients taking insulin. In the United States alone, approximately 300,000 people are hospitalized for the disease each year.
Dasiglucagon is a world-first soluble stable analog of glucagon discovered and developed by Zealand. Glucagon is a hormone secreted by the islet α-cells of the pancreas. It is opposed to insulin and plays a role in increasing blood sugar. Zealand uses its unique peptide drug research platform to change some of the amino acids in wild-type glucagon to maintain its biological activity and make it have higher stability and solubility. Compared with the previously marketed lyophilized powder preparation therapeutic drugs, it can be pre-installed in the emergency pen and can be injected subcutaneously during clinical use, which is extremely convenient.
The FDA approval was mainly based on the efficacy data of three randomized, double-blind, placebo-controlled, multi-center phase III trials conducted in adults with type 1 diabetes between the ages of 6 and 17 years. The data shows that whether it is an adult patient or a child patient, the median time for blood glucose levels to recover after receiving Dasiglucagon injection is 10 minutes, while that of the placebo control group is 30 to 45 minutes. In a phase III study for adults, 99% of hypoglycemia patients recovered from insulin-induced hypoglycemia within 15 minutes after using Dasiglucagon. The study confirmed the potential of Dasiglucagon to quickly and effectively rescue severe hypoglycemia in diabetic patients.
Dasiglucagon expects to start marketing the drug in the United States in June, bringing new treatment options to diabetic patients. It must be said that this is an important milestone in the history of diabetic hypoglycemia treatment. Let us look forward to it!
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2021-03-25 14:09
- 访问量:
On March 22, the Danish pharmaceutical company Zealand Pharma, established in 1998, announced that the FDA approved Dasiglucagon subcutaneous injections for the treatment of severe hypoglycemia in children over 6 years old and adults with diabetes.
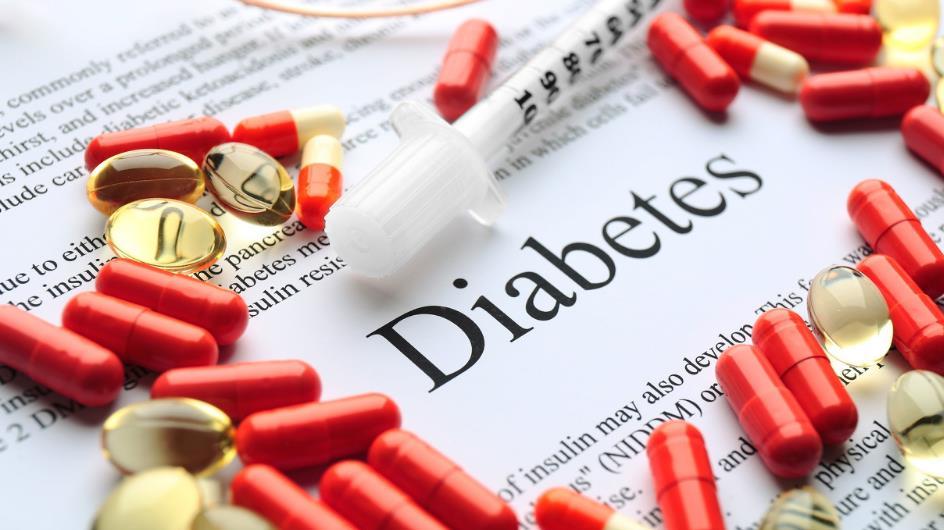
Hypoglycemia is caused by a severe drop in blood sugar levels, and severe hypoglycemia is generally an acute and life-threatening condition in which the blood sugar level suddenly drops when diabetic patients receive insulin therapy. Severe hypoglycemia is also the most typical complication in the clinical treatment of diabetes. It mainly occurs in type 1 diabetic patients and type 2 diabetic patients taking insulin. In the United States alone, approximately 300,000 people are hospitalized for the disease each year.
Dasiglucagon is a world-first soluble stable analog of glucagon discovered and developed by Zealand. Glucagon is a hormone secreted by the islet α-cells of the pancreas. It is opposed to insulin and plays a role in increasing blood sugar. Zealand uses its unique peptide drug research platform to change some of the amino acids in wild-type glucagon to maintain its biological activity and make it have higher stability and solubility. Compared with the previously marketed lyophilized powder preparation therapeutic drugs, it can be pre-installed in the emergency pen and can be injected subcutaneously during clinical use, which is extremely convenient.

The FDA approval was mainly based on the efficacy data of three randomized, double-blind, placebo-controlled, multi-center phase III trials conducted in adults with type 1 diabetes between the ages of 6 and 17 years. The data shows that whether it is an adult patient or a child patient, the median time for blood glucose levels to recover after receiving Dasiglucagon injection is 10 minutes, while that of the placebo control group is 30 to 45 minutes. In a phase III study for adults, 99% of hypoglycemia patients recovered from insulin-induced hypoglycemia within 15 minutes after using Dasiglucagon. The study confirmed the potential of Dasiglucagon to quickly and effectively rescue severe hypoglycemia in diabetic patients.
Dasiglucagon expects to start marketing the drug in the United States in June, bringing new treatment options to diabetic patients. It must be said that this is an important milestone in the history of diabetic hypoglycemia treatment. Let us look forward to it!


美国联邦巡回上诉法院表示娇生公司 全球畅销精神分裂症药物的专利可能无效

注射用醋酸地加瑞克专利无效口审将于本月底进行

外观专利如何进行海外布局?