NEWS
最新消息
Inmazeb, the First FDA Approved Treatment for Ebola Virus
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2020-10-22 16:27
- 访问量:
【概要描述】On October 14, the U.S. Food and Drug Administration approved Regeneron’s Inmazeb as the first FDA-approved treatment for Ebola virus infection in adult and pediatric patients. Zaire ebolavirus, commonly known as Ebola virus, can cause a potentially fatal human disease. Ebola virus is transmitted through direct contact with blood, body fluids and tissues of infected people or wild animals, as well as with surfaces and materials, such as bedding and clothing, contaminated with these fluids. Individuals who provide care for people with Ebola virus, including health care workers who do not use correct infection control precautions, are at the highest risk for infection. Ebola is the cause of a viral hemorrhagic fever disease (Ebola virus disease, or EVD). The viruses that cause EVD are well known to be located mainly in sub-Saharan Africa. Inmazeb, formerly known as REGN-EB3, is developed by Regeneron Pharmaceuticals, and is a mixture of the following three monoclonal antibodies: atoltivimab, maftivimab, and odesivimab-ebgn. Inmazeb targets the glycoprotein that is on the surface of Ebola virus. Glycoprotein attaches to the cell receptor and fuses the viral and host cell membranes allowing the virus to enter the cell. The three antibodies that make up Inmazeb can bind to this glycoprotein simultaneously and block attachment and entry of the virus. Inmazeb once received an Orphan Drug designation for the treatment of Ebola virus infection. Additionally, the FDA previously granted Inmazeb a Breakthrough Therapy designation for the treatment of Zaire ebolavirus infection. “Today’s approval highlights the importance of international collaboration in the fight against Ebola virus,” said John Farley, M.D., MPH, director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research. “The urgent need for advanced therapies to combat this infectious disease is clear, and today’s action is a significant step forward in that effort.”
Inmazeb, the First FDA Approved Treatment for Ebola Virus
【概要描述】On October 14, the U.S. Food and Drug Administration approved Regeneron’s Inmazeb as the first FDA-approved treatment for Ebola virus infection in adult and pediatric patients.
Zaire ebolavirus, commonly known as Ebola virus, can cause a potentially fatal human disease. Ebola virus is transmitted through direct contact with blood, body fluids and tissues of infected people or wild animals, as well as with surfaces and materials, such as bedding and clothing, contaminated with these fluids. Individuals who provide care for people with Ebola virus, including health care workers who do not use correct infection control precautions, are at the highest risk for infection. Ebola is the cause of a viral hemorrhagic fever disease (Ebola virus disease, or EVD). The viruses that cause EVD are well known to be located mainly in sub-Saharan Africa.
Inmazeb, formerly known as REGN-EB3, is developed by Regeneron Pharmaceuticals, and is a mixture of the following three monoclonal antibodies: atoltivimab, maftivimab, and odesivimab-ebgn. Inmazeb targets the glycoprotein that is on the surface of Ebola virus. Glycoprotein attaches to the cell receptor and fuses the viral and host cell membranes allowing the virus to enter the cell. The three antibodies that make up Inmazeb can bind to this glycoprotein simultaneously and block attachment and entry of the virus.
Inmazeb once received an Orphan Drug designation for the treatment of Ebola virus infection. Additionally, the FDA previously granted Inmazeb a Breakthrough Therapy designation for the treatment of Zaire ebolavirus infection.
“Today’s approval highlights the importance of international collaboration in the fight against Ebola virus,” said John Farley, M.D., MPH, director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research. “The urgent need for advanced therapies to combat this infectious disease is clear, and today’s action is a significant step forward in that effort.”
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2020-10-22 16:27
- 访问量:
On October 14, the U.S. Food and Drug Administration approved Regeneron’s Inmazeb as the first FDA-approved treatment for Ebola virus infection in adult and pediatric patients.
Zaire ebolavirus, commonly known as Ebola virus, can cause a potentially fatal human disease. Ebola virus is transmitted through direct contact with blood, body fluids and tissues of infected people or wild animals, as well as with surfaces and materials, such as bedding and clothing, contaminated with these fluids. Individuals who provide care for people with Ebola virus, including health care workers who do not use correct infection control precautions, are at the highest risk for infection. Ebola is the cause of a viral hemorrhagic fever disease (Ebola virus disease, or EVD). The viruses that cause EVD are well known to be located mainly in sub-Saharan Africa.
Inmazeb, formerly known as REGN-EB3, is developed by Regeneron Pharmaceuticals, and is a mixture of the following three monoclonal antibodies: atoltivimab, maftivimab, and odesivimab-ebgn. Inmazeb targets the glycoprotein that is on the surface of Ebola virus. Glycoprotein attaches to the cell receptor and fuses the viral and host cell membranes allowing the virus to enter the cell. The three antibodies that make up Inmazeb can bind to this glycoprotein simultaneously and block attachment and entry of the virus.
Inmazeb once received an Orphan Drug designation for the treatment of Ebola virus infection. Additionally, the FDA previously granted Inmazeb a Breakthrough Therapy designation for the treatment of Zaire ebolavirus infection.
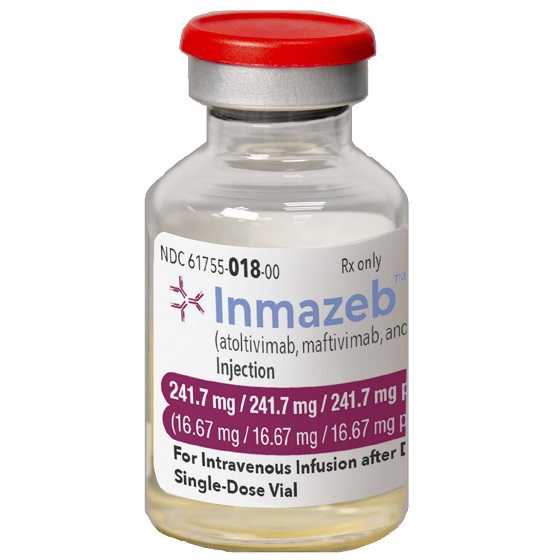
“Today’s approval highlights the importance of international collaboration in the fight against Ebola virus,” said John Farley, M.D., MPH, director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research. “The urgent need for advanced therapies to combat this infectious disease is clear, and today’s action is a significant step forward in that effort.”


美国联邦巡回上诉法院表示娇生公司 全球畅销精神分裂症药物的专利可能无效

注射用醋酸地加瑞克专利无效口审将于本月底进行

外观专利如何进行海外布局?