NEWS
最新消息
Guardant 360 CDx the First FDA Approved NGS Liquid Biopsy Product
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2020-08-21 15:12
- 访问量:
【概要描述】With the increasing incidence and mortality of tumors, the rapid and effective diagnosis and treatment of tumors have become the focus of the precision medicine industry. In clinical practice, the limitation of tissue samples has been proved to be a major obstacle to the promotion of precision medicine in oncology. It has been estimated that up to 30% of patients with advanced cancer cannot carry out gene mutation analysis by tumor tissue NGS detection to optimize treatment decisions due to insufficient or unavailable tissue samples. In this case, patients can use the biomarker information in blood and other body fluids to guide cancer treatment decisions. Recently, U.S. Food and Drug Administration (FDA) approved Guardant Health's Guardant 360 CDx for comprehensive genomic analysis of all solid tumor types. Meanwhile, FDA has approved Guardant 360 CDx as an accompanying test for the identification of patients with metastatic non-small cell lung cancer (NSCLC) carrying epidermal growth factor receptor (EGFR) gene mutations. Guardant 360 CDx is the first FDA approved diagnostic test that combines NGS and liquid biopsy techniques to guide clinical treatment decisions. Guardant 360 CDx detection mainly uses two techniques. The first is liquid biopsy. Compared with standard tissue biopsy, it is less invasive and easy to repeat, and is suitable for tumor patients who are unable to carry out standard tissue biopsy. The second technology is NGS. Guardant 360 CDx uses large panel gene sequencing technology for high-throughput tumor analysis, which can detect mutations of 55 tumor genes at the same time. Compared with the earlier detection technology, this test only needs one test, which can help clinicians better evaluate tumor gene mutation and provide better treatment decision for patients. It shortens the waiting time for patients to receive treatment and deeply understand the possible mechanisms of drug resistance. “FDA's approval of Guardant 360 CDx is a landmark decision that demonstrates the value of liquid biopsy for oncologists and cancer patients.” said Dr Helmy Eltoukhy, CEO of Guardian Health. FDA approval will help accelerate the wider adoption of genomic analysis recommended by the guidelines, enable more advanced cancer patients to receive better treatment, and promote precision medicine to a new era.
Guardant 360 CDx the First FDA Approved NGS Liquid Biopsy Product
【概要描述】With the increasing incidence and mortality of tumors, the rapid and effective diagnosis and treatment of tumors have become the focus of the precision medicine industry. In clinical practice, the limitation of tissue samples has been proved to be a major obstacle to the promotion of precision medicine in oncology. It has been estimated that up to 30% of patients with advanced cancer cannot carry out gene mutation analysis by tumor tissue NGS detection to optimize treatment decisions due to insufficient or unavailable tissue samples. In this case, patients can use the biomarker information in blood and other body fluids to guide cancer treatment decisions.
Recently, U.S. Food and Drug Administration (FDA) approved Guardant Health's Guardant 360 CDx for comprehensive genomic analysis of all solid tumor types. Meanwhile, FDA has approved Guardant 360 CDx as an accompanying test for the identification of patients with metastatic non-small cell lung cancer (NSCLC) carrying epidermal growth factor receptor (EGFR) gene mutations. Guardant 360 CDx is the first FDA approved diagnostic test that combines NGS and liquid biopsy techniques to guide clinical treatment decisions.
Guardant 360 CDx detection mainly uses two techniques. The first is liquid biopsy. Compared with standard tissue biopsy, it is less invasive and easy to repeat, and is suitable for tumor patients who are unable to carry out standard tissue biopsy. The second technology is NGS. Guardant 360 CDx uses large panel gene sequencing technology for high-throughput tumor analysis, which can detect mutations of 55 tumor genes at the same time. Compared with the earlier detection technology, this test only needs one test, which can help clinicians better evaluate tumor gene mutation and provide better treatment decision for patients. It shortens the waiting time for patients to receive treatment and deeply understand the possible mechanisms of drug resistance.
“FDA's approval of Guardant 360 CDx is a landmark decision that demonstrates the value of liquid biopsy for oncologists and cancer patients.” said Dr Helmy Eltoukhy, CEO of Guardian Health. FDA approval will help accelerate the wider adoption of genomic analysis recommended by the guidelines, enable more advanced cancer patients to receive better treatment, and promote precision medicine to a new era.
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2020-08-21 15:12
- 访问量:
With the increasing incidence and mortality of tumors, the rapid and effective diagnosis and treatment of tumors have become the focus of the precision medicine industry. In clinical practice, the limitation of tissue samples has been proved to be a major obstacle to the promotion of precision medicine in oncology. It has been estimated that up to 30% of patients with advanced cancer cannot carry out gene mutation analysis by tumor tissue NGS detection to optimize treatment decisions due to insufficient or unavailable tissue samples. In this case, patients can use the biomarker information in blood and other body fluids to guide cancer treatment decisions.

Recently, U.S. Food and Drug Administration (FDA) approved Guardant Health's Guardant 360 CDx for comprehensive genomic analysis of all solid tumor types. Meanwhile, FDA has approved Guardant 360 CDx as an accompanying test for the identification of patients with metastatic non-small cell lung cancer (NSCLC) carrying epidermal growth factor receptor (EGFR) gene mutations. Guardant 360 CDx is the first FDA approved diagnostic test that combines NGS and liquid biopsy techniques to guide clinical treatment decisions.
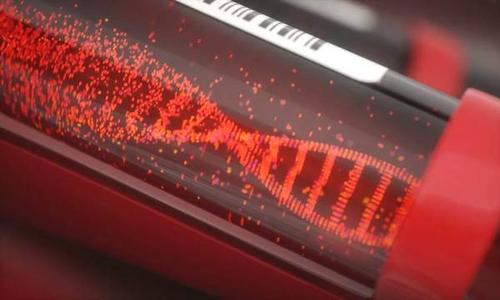
Guardant 360 CDx detection mainly uses two techniques. The first is liquid biopsy. Compared with standard tissue biopsy, it is less invasive and easy to repeat, and is suitable for tumor patients who are unable to carry out standard tissue biopsy. The second technology is NGS. Guardant 360 CDx uses large panel gene sequencing technology for high-throughput tumor analysis, which can detect mutations of 55 tumor genes at the same time. Compared with the earlier detection technology, this test only needs one test, which can help clinicians better evaluate tumor gene mutation and provide better treatment decision for patients. It shortens the waiting time for patients to receive treatment and deeply understand the possible mechanisms of drug resistance.

“FDA's approval of Guardant 360 CDx is a landmark decision that demonstrates the value of liquid biopsy for oncologists and cancer patients.” said Dr Helmy Eltoukhy, CEO of Guardian Health. FDA approval will help accelerate the wider adoption of genomic analysis recommended by the guidelines, enable more advanced cancer patients to receive better treatment, and promote precision medicine to a new era.