NEWS
最新消息
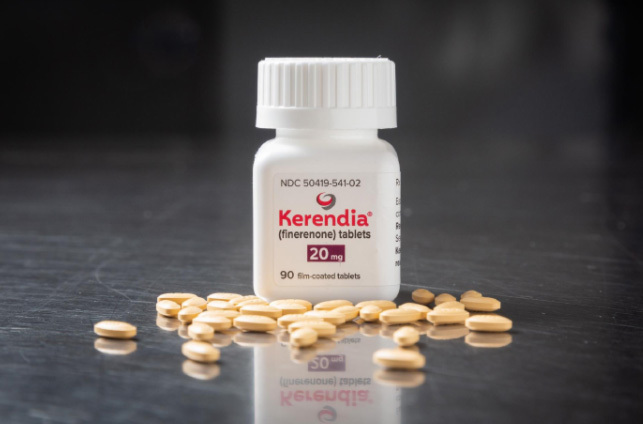
Kerendia Receives FDA Approval for Slowing chronic kidney disease in Type 2 Diabetes
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2021-07-15 11:00
- 访问量:
【概要描述】Kerendia Receives FDA Approval for Slowing chronic kidney disease in Type 2 Diabetes This month FDA announced that they have approved Kerendia (finerenone) tablets for the treatment of patients with chronic kidney disease associated with type 2 diabetes. Kerendia is indicated to delay chronic kidney disease progression. It is able to reduce the risk of kidney function decline, kidney failure, cardiovascular death, non-fatal heart attacks, and hospitalization for heart failure in adult diabetic patients. Now the drug has also been submitted for marketing authorization in China, European Union and other countries. There are 422 million people with diabetes, and there are up to 40% of all patients with type 2 diabetes develop chronic kidney disease. Diabetes is one of the most common causes of chronic kidney disease and kidney failure. Chronic kidney disease is the gradual loss of kidney function over a period of months to years. It occurs when the kidneys are damaged and cannot filter blood normally, and sometimes can progress to kidney failure. The patients are also at high risk of heart disease because of defective filtering. Kerendia is the first and only nonsteroidal mineralocorticoid receptor antagonist (MRA) for Type 2 diabetic patients with chronic kidney disease, compared to SGLT2 inhibitors, Farxiga (dapagliflozin) and Invokana (canagliflozin). It blocks overactivation of the mineralocorticoid receptor (MR) which contributes to fibrosis and inflammation. The drug is able to target fibrosis and inflammation to slow kidney disease progression. The efficacy of Kerendia was evaluated in a randomized, multicenter, double-blind, placebo-controlled in the phase III trial. There were 5,674 patients in the study. The study found that Kerendia beat placebo when it came to kidney function. That included fending off or slowing progression to kidney failure and kidney death. Furthermore, the study also found that Kerendia reduced the risk of cardiovascular death, non-fatal heart attack, and hospitalization for heart failure. Kerendia received priority review and fast track designations for their application, and it will be commercially available in the U.S. by the end of July. 資料來源: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-drug-reduce-risk-serious-kidney-and-heart-complications-adults-chronic-kidney-disease https://www.pharmalive.com/bayers-kerendia-receives-u-s-fda-approval/ https://finance.yahoo.com/news/bayers-kerendia-scores-long-awaited-111105329.html https://www.businesswire.com/news/home/20210709005441/en/Bayer%E2%80%99s-KERENDIA%C2%AE-finerenone-Receives-U.S.-FDA-Approval-for-Treatment-of-Patients-with-Chronic-Kidney-Disease-Associated-with-Type-2-Diabetes https://www.healio.com/news/endocrinology/20200710/finerenone-delays-diabetic-kidney-disease-progression-fideliodkd
Kerendia Receives FDA Approval for Slowing chronic kidney disease in Type 2 Diabetes
【概要描述】Kerendia Receives FDA Approval for Slowing chronic kidney disease in Type 2 Diabetes
This month FDA announced that they have approved Kerendia (finerenone) tablets for the treatment of patients with chronic kidney disease associated with type 2 diabetes. Kerendia is indicated to delay chronic kidney disease progression. It is able to reduce the risk of kidney function decline, kidney failure, cardiovascular death, non-fatal heart attacks, and hospitalization for heart failure in adult diabetic patients. Now the drug has also been submitted for marketing authorization in China, European Union and other countries.
There are 422 million people with diabetes, and there are up to 40% of all patients with type 2 diabetes develop chronic kidney disease. Diabetes is one of the most common causes of chronic kidney disease and kidney failure. Chronic kidney disease is the gradual loss of kidney function over a period of months to years. It occurs when the kidneys are damaged and cannot filter blood normally, and sometimes can progress to kidney failure. The patients are also at high risk of heart disease because of defective filtering.
Kerendia is the first and only nonsteroidal mineralocorticoid receptor antagonist (MRA) for Type 2 diabetic patients with chronic kidney disease, compared to SGLT2 inhibitors, Farxiga (dapagliflozin) and Invokana (canagliflozin). It blocks overactivation of the mineralocorticoid receptor (MR) which contributes to fibrosis and inflammation. The drug is able to target fibrosis and inflammation to slow kidney disease progression.
The efficacy of Kerendia was evaluated in a randomized, multicenter, double-blind, placebo-controlled in the phase III trial. There were 5,674 patients in the study. The study found that Kerendia beat placebo when it came to kidney function. That included fending off or slowing progression to kidney failure and kidney death. Furthermore, the study also found that Kerendia reduced the risk of cardiovascular death, non-fatal heart attack, and hospitalization for heart failure.
Kerendia received priority review and fast track designations for their application, and it will be commercially available in the U.S. by the end of July.
資料來源:
https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-drug-reduce-risk-serious-kidney-and-heart-complications-adults-chronic-kidney-disease
https://www.pharmalive.com/bayers-kerendia-receives-u-s-fda-approval/
https://finance.yahoo.com/news/bayers-kerendia-scores-long-awaited-111105329.html
https://www.businesswire.com/news/home/20210709005441/en/Bayer%E2%80%99s-KERENDIA%C2%AE-finerenone-Receives-U.S.-FDA-Approval-for-Treatment-of-Patients-with-Chronic-Kidney-Disease-Associated-with-Type-2-Diabetes
https://www.healio.com/news/endocrinology/20200710/finerenone-delays-diabetic-kidney-disease-progression-fideliodkd
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2021-07-15 11:00
- 访问量:
Kerendia Receives FDA Approval for Slowing chronic kidney disease in Type 2 Diabetes
This month FDA announced that they have approved Kerendia (finerenone) tablets for the treatment of patients with chronic kidney disease associated with type 2 diabetes. Kerendia is indicated to delay chronic kidney disease progression. It is able to reduce the risk of kidney function decline, kidney failure, cardiovascular death, non-fatal heart attacks, and hospitalization for heart failure in adult diabetic patients. Now the drug has also been submitted for marketing authorization in China, European Union and other countries.
There are 422 million people with diabetes, and there are up to 40% of all patients with type 2 diabetes develop chronic kidney disease. Diabetes is one of the most common causes of chronic kidney disease and kidney failure. Chronic kidney disease is the gradual loss of kidney function over a period of months to years. It occurs when the kidneys are damaged and cannot filter blood normally, and sometimes can progress to kidney failure. The patients are also at high risk of heart disease because of defective filtering.
Kerendia is the first and only nonsteroidal mineralocorticoid receptor antagonist (MRA) for Type 2 diabetic patients with chronic kidney disease, compared to SGLT2 inhibitors, Farxiga (dapagliflozin) and Invokana (canagliflozin). It blocks overactivation of the mineralocorticoid receptor (MR) which contributes to fibrosis and inflammation. The drug is able to target fibrosis and inflammation to slow kidney disease progression.
The efficacy of Kerendia was evaluated in a randomized, multicenter, double-blind, placebo-controlled in the phase III trial. There were 5,674 patients in the study. The study found that Kerendia beat placebo when it came to kidney function. That included fending off or slowing progression to kidney failure and kidney death. Furthermore, the study also found that Kerendia reduced the risk of cardiovascular death, non-fatal heart attack, and hospitalization for heart failure.
Kerendia received priority review and fast track designations for their application, and it will be commercially available in the U.S. by the end of July.
資料來源:
- https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-drug-reduce-risk-serious-kidney-and-heart-complications-adults-chronic-kidney-disease
- https://www.pharmalive.com/bayers-kerendia-receives-u-s-fda-approval/
- https://finance.yahoo.com/news/bayers-kerendia-scores-long-awaited-111105329.html
- https://www.businesswire.com/news/home/20210709005441/en/Bayer%E2%80%99s-KERENDIA%C2%AE-finerenone-Receives-U.S.-FDA-Approval-for-Treatment-of-Patients-with-Chronic-Kidney-Disease-Associated-with-Type-2-Diabetes
- https://www.healio.com/news/endocrinology/20200710/finerenone-delays-diabetic-kidney-disease-progression-fideliodkd


美国联邦巡回上诉法院表示娇生公司 全球畅销精神分裂症药物的专利可能无效

注射用醋酸地加瑞克专利无效口审将于本月底进行

外观专利如何进行海外布局?