NEWS
最新消息
FDA Approval of CYRAMZA’s New Indication
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2020-06-12 14:43
- 访问量:
【概要描述】OnMay29,2020,aPriorApprovalsupplementalbiologicsapplicationwasapprovedbytheFDA,whichapplicationprovidesforanewindicationofCYRAMZATM(activeingredient:ramucirumab),incombinationwitherlotinib(TarcevaTM),
FDA Approval of CYRAMZA’s New Indication
【概要描述】OnMay29,2020,aPriorApprovalsupplementalbiologicsapplicationwasapprovedbytheFDA,whichapplicationprovidesforanewindicationofCYRAMZATM(activeingredient:ramucirumab),incombinationwitherlotinib(TarcevaTM),
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2020-06-12 14:43
- 访问量:
On May 29, 2020, a Prior Approval supplemental biologics application was approved by the FDA, which application provides for a new indication of CYRAMZATM (active ingredient: ramucirumab), in combination with erlotinib (TarcevaTM), for first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) mutations.
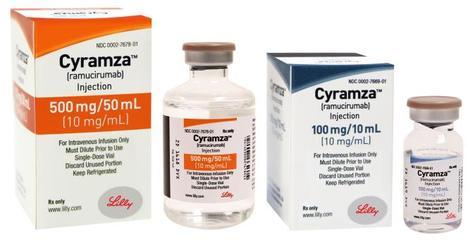
CYRAMZA is a human vascular endothelial growth factor receptor 2 (VEGFR2) antagonist produced by Eli Lilly and Company. This combination is now the first and only FDA-approved anti-VEGFR/EGFR TKI combination therapy for this patient population. With this approval, CYRAMZA has now received the 6 FDA approvals to treat certain types of lung, liver, stomach, and colorectal cancers; in particular, gastric cancer, non-small cell lung cancer, colorectal cancer, and hepatocellular carcinoma.
“We’re encouraged by (CYRAMZA’s) latest approval, which represents one step towards our goal of making EGFR-mutated NSCLC in a manageable chronic disease,” Ivy Elkins, cofounder of EGFR Resisters, said in a press release. “Each new treatment options gives hope to those living with this disease and provides oncologists with more options that may help slow the spread of this deadly cancer, which is an important goal for many patients.”
About 50% of patients with lung cancer are diagnosed with advanced disease or metastasis. The five-year survival rate of patients with metastatic NSCLC is 6%. Approximately 15% of American NSCLC patients carry EGFR mutations. Therefore, as Edward Garon, North America lead investigator of the (CYRAMZA’s) phase III RELAY trial stated, the approval of this new first-line metastatic EGFR-mutated NSCLC regiment, which inhibits the VEGFR and EGFR pathways together, is an important milestone in the treatment of this disease. Patients now have multiple options for initial therapy capable of delaying disease progression for considerably longer than erlotinib, which has been traditional standard approach.
Outside the United States, Eli Lilly has submitted a CYRAMZA marketing application in Japan based on the results of the phase III RELAY study, which is expected to be approved by the end of 2020.


美国联邦巡回上诉法院表示娇生公司 全球畅销精神分裂症药物的专利可能无效

注射用醋酸地加瑞克专利无效口审将于本月底进行

外观专利如何进行海外布局?