NEWS
最新消息
AstraZeneca files patent infringement lawsuits in China
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2019-06-28 16:54
- 访问量:
【概要描述】ItwasreportedbyChinaIPMagazinethatAstraZenecahasfiledtwopatentinfringementlawsuitsagainstgenericdrugcompaniesJiangsuOsaikangPharmaceuticalCo.,Ltd.andChongqingHuabangPharmaceuticalCo.,Ltd.onApril23andA
AstraZeneca files patent infringement lawsuits in China
【概要描述】ItwasreportedbyChinaIPMagazinethatAstraZenecahasfiledtwopatentinfringementlawsuitsagainstgenericdrugcompaniesJiangsuOsaikangPharmaceuticalCo.,Ltd.andChongqingHuabangPharmaceuticalCo.,Ltd.onApril23andA
- 分类:最新消息
- 作者:华讯知识产权
- 来源:
- 发布时间:2019-06-28 16:54
- 访问量:
It was reported by China IP Magazine that AstraZeneca has filed two patent infringement lawsuits against generic drug companies Jiangsu Osaikang Pharmaceutical Co., Ltd. and Chongqing Huabang Pharmaceutical Co., Ltd. on April 23 and April 24, respectively, for an antidiabetic drug Saxagliptin (Enrizel) and a breast cancer drug Anastrazole (Reningde).

Saxagliptin
Trade Name: Enrizel (ONGLYZA™)
Structural Formula: C18H25N3O2
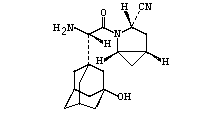
Saglitine tablets, a dipeptidyl Peptidase 4 (DPP-4) inhibitor developed by Bristol-Myers Squibb Company, can increase the levels of endogenous glucagon-like Peptide-1 (GLP-1) and glucose-dependent insulin-releasing polypeptide (GIP), thus regulating blood sugar to treat Type 2 diabetes. The drug was first approved for marketing in the United States in 2009, then acquired by AstraZeneca, and approved for marketing in China in 2011. In 2017, Shaglitin, together with other four DDP-4 inhibitors, was included in the National Reimbursement Drug List for the first time. At present, only AstraZeneca is producing and selling the drug in the domestic market. The generic drug of Shaglistine by Aosaikang Pharmaceutical was approved for marketing by the National Medical Products Administration in January 2019 and is the first one in China to pass a consistent evaluation.
In the lawsuit against Aosaikang Pharmaceutical, AstraZeneca alleged that Saxagliptin Tablets by the former fell within the protection scope of its invention patent CN1213028C, which is expected to expire on March 5, 2021. AstraZeneca petitioned the court to order Osaka not to infringe on its patent rights during the validity period of the said patent, i.e., not to manufacture, use, sell and promise to sell infringing products during the validity period of the patent.
|
Publication No. |
Patent Name |
Application Date |
Patent Date |
|
CN1213028C |
Dipeptidyl peptidase IV inhibitors based on cyclopropyl-fused pyrrolidine, their preparation methods and applications |
2001-03-05 |
2005-08-03 |
Anastrozole
Trade Name: Arimidex®
Structural Formula: C17H19N5
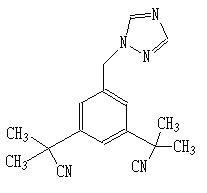
 Anastrazole Tablets, developed by AstraZeneca, are mainly used as adjuvant therapy for early breast cancer cases with positive hormone receptor in post-menopausal women. AstraZeneca alleged that its Anastrazole tablets product Arimidex was still protected by the invention patent CN100408036C and Ruiting by Chongqing Huapont Pharm, fell within the protection scope of the said patent. The patent for Arimidex, which is about indications and methods, involves indications for early breast cancer cases except for advanced breast cancer, and is expected to expire on December 16, 2022. AstraZeneca petitioned the court to require Chongqing Huapont Pharm not to infringe its patent right during the validity period of the patent.
Anastrazole Tablets, developed by AstraZeneca, are mainly used as adjuvant therapy for early breast cancer cases with positive hormone receptor in post-menopausal women. AstraZeneca alleged that its Anastrazole tablets product Arimidex was still protected by the invention patent CN100408036C and Ruiting by Chongqing Huapont Pharm, fell within the protection scope of the said patent. The patent for Arimidex, which is about indications and methods, involves indications for early breast cancer cases except for advanced breast cancer, and is expected to expire on December 16, 2022. AstraZeneca petitioned the court to require Chongqing Huapont Pharm not to infringe its patent right during the validity period of the patent.
|
Publication No. |
Patent Name |
Application Date |
Patent Date |
|
CN100408036C |
The use of Anastrozole in the preparation of drugs for post-menopausal women with early breast cancer |
2002-12-06 |
2008-08-06 |
Summary
According to statistics, there are 84 patents for drugs approved for marketing in China since 2000, which have expired or will expire between 2018 and 2022. In order to market generic drugs immediately after patents expire, domestic enterprises have already made overall arrangements in advance. In case that a patent of a pharmaceutical compound has not expired and is still valid, it has become a trend to get the approval of the National Medical Products Administration for marketing the related generic drugs in advance. When there is no effective drug patent linkage system in China, it is predictable that drug patent infringement lawsuits will probably surge.
References:https://med.sina.com/article_detail_103_2_65266.html


美国联邦巡回上诉法院表示娇生公司 全球畅销精神分裂症药物的专利可能无效

注射用醋酸地加瑞克专利无效口审将于本月底进行

外观专利如何进行海外布局?